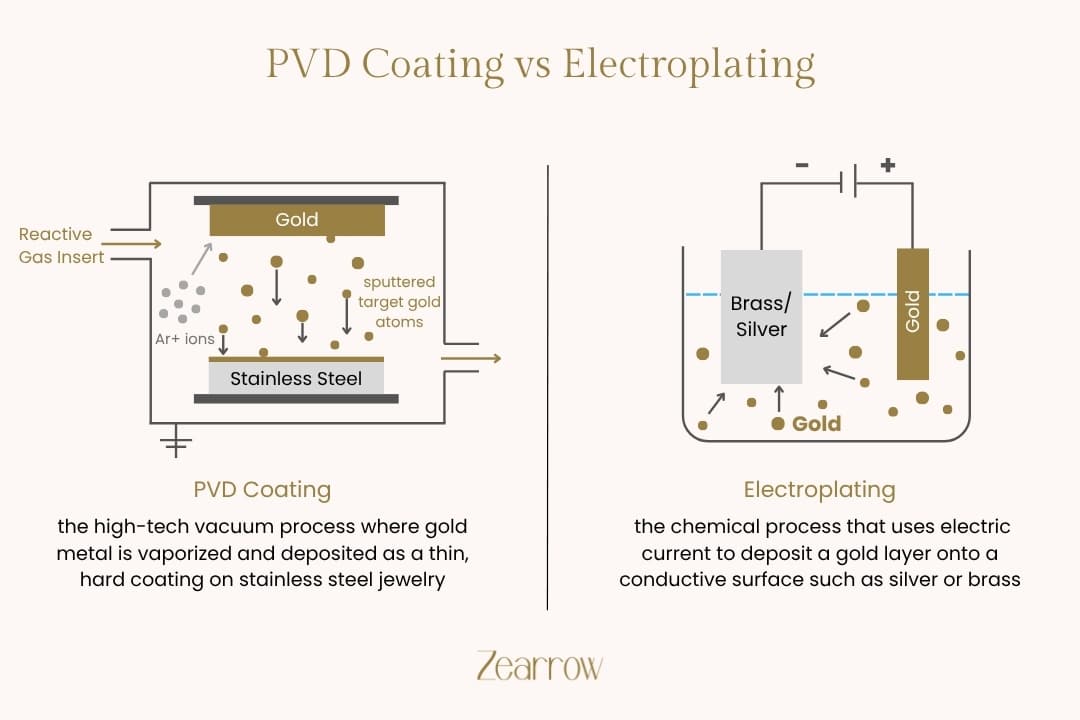
The Critical Choice in Surface Engineering: Electroplating Versus PVD Coating
As a Manufacturing Technician, my focus is always on the intersection of material science, process control, and final product integrity. When we talk about durable, aesthetically pleasing corporate gifts—be it a premium metal pen or a custom-plated power bank casing—the surface finish is not merely a cosmetic detail; it is a functional layer that dictates longevity, corrosion resistance, and perceived value. The two dominant players in this arena are traditional Electroplating (EP) and the more advanced Physical Vapour Deposition (PVD). Understanding the fundamental differences in their deposition mechanisms and process parameters is crucial for achieving optimal results and managing production costs in our Malaysian facilities.
Electroplating: The Electrochemical Foundation
Electroplating is a mature, well-understood process rooted in electrochemistry. It involves reducing metal cations from a solution and depositing them as a thin, coherent metal coating onto a conductive substrate. The workpiece, or substrate, acts as the cathode, while the anode is typically the plating metal itself or an inert material. The entire system is immersed in an electrolyte bath, and a direct current is applied.
The process is deceptively simple, yet its control demands rigorous attention to detail. The initial substrate preparation is paramount. Any residual oils, oxides, or particulates will compromise the adhesion and uniformity of the final deposit. This typically involves a multi-stage cleaning sequence: solvent degreasing, alkaline cleaning, acid activation, and multiple rinsing stages. A failure in this stage is the primary cause of blistering and poor adhesion, leading to costly rework and reduced yield rates.
Process Control and Technical Parameters
In the plating tank, several critical parameters must be meticulously monitored. Current density (A/dm²) is the most influential variable, directly controlling the deposition rate and the morphology of the coating. Too low, and the process is inefficient; too high, and you risk "burning" the deposit, resulting in a rough, non-uniform, and brittle layer. The bath chemistry, including the concentration of the metal salt, pH level, and the presence of brighteners and levellers, must be maintained within tight tolerances. These additives are essential for achieving the desired aesthetic properties, such as a mirror-bright finish.
One of the inherent challenges of EP is its throwing power, or the ability of the process to deposit a uniform coating on complex geometries. Due to the nature of the electric field, current density is highest on sharp edges and external corners, leading to thicker deposits in these areas and thinner, sometimes insufficient, coverage in recessed areas (low current density regions). This non-uniformity requires careful jigging and sometimes the use of auxiliary anodes to ensure acceptable coverage, especially for intricate corporate gift designs.
From a material science perspective, EP deposits are typically softer and more porous than PVD coatings. While post-treatment processes like chromate conversion or clear lacquering can enhance corrosion resistance, the inherent structure of the electrodeposited layer means it is more susceptible to wear and tear over time. For high-volume production of items like promotional keychains or badges, EP offers a lower capital expenditure (CAPEX) and high throughput, making it a cost-effective solution, provided the required durability is moderate. When discussing the economics of large-scale production, understanding the trade-offs between initial setup cost and long-term operational expenses is key, which is why we often refer to the principles of navigating Minimum Order Quantities (MOQs) to determine the most viable finishing route.
Physical Vapour Deposition: The High-Vacuum Solution
Physical Vapour Deposition, or PVD, operates on an entirely different principle: a high-vacuum coating process where the coating material is vaporised and then deposited atom-by-atom onto the substrate. This is a line-of-sight process conducted within a sealed vacuum chamber, typically at pressures below $10^{-2}$ Pascal. The two most common PVD techniques relevant to our industry are sputtering and arc evaporation.
In sputtering, high-energy ions (usually Argon) are accelerated towards a solid target material (the source of the coating). The impact physically knocks off, or "sputters," atoms from the target, which then travel through the vacuum and condense on the cooler substrate. Arc evaporation uses a high-current, low-voltage arc to vaporise the target material, creating a highly ionised plasma that is then directed towards the substrate.
The Rigours of Vacuum Integrity
The success of PVD hinges on maintaining absolute vacuum integrity and precise control over the plasma environment. Substrate preparation is still critical, but the final cleaning is often performed in-situ within the vacuum chamber using plasma etching or ion bombardment. This final cleaning step ensures a pristine surface, which is essential for the superior adhesion that PVD is known for. The high kinetic energy of the depositing atoms allows them to penetrate the substrate surface slightly, creating a strong diffusion bond rather than the purely mechanical or weak metallic bond often seen in EP.
The technical parameters in PVD are centred around vacuum level, gas flow (e.g., Argon for sputtering, Nitrogen for reactive deposition), power input (DC, RF, or pulsed), and substrate temperature. Unlike EP, PVD coatings are highly uniform, even on complex shapes, provided the parts are rotated or the vapour source is strategically placed. The line-of-sight nature means that any area shielded from the vapour stream will not be coated, which is a design consideration we must always factor in when planning for tech accessories and complex casings.
PVD coatings are characteristically harder, denser, and more chemically inert than electroplated layers. Common PVD materials like Titanium Nitride (TiN), Zirconium Nitride (ZrN), and Chromium Nitride (CrN) offer exceptional wear resistance and a range of vibrant, non-fading colours, which is a significant advantage for premium corporate gifts destined for long-term use.
A Comparative Analysis: Performance and Process Economics
The decision between EP and PVD is rarely straightforward; it is a calculated risk assessment based on the required performance envelope, the substrate material, and the project's budget.
| Feature | Electroplating (EP) | Physical Vapour Deposition (PVD) | Technician's Assessment |
|---|---|---|---|
| Deposition Mechanism | Electrochemical reduction in liquid electrolyte. | Atom-by-atom condensation from vapour phase in high vacuum. | PVD offers superior control over atomic structure. |
| Adhesion | Good (primarily mechanical/metallic bond). | Excellent (strong diffusion bond due to high kinetic energy). | PVD's bond strength is fundamentally superior for high-stress applications. |
| Coating Hardness | Low to Moderate (e.g., soft gold, nickel). | High to Very High (e.g., TiN, CrN, DLC). | PVD is the clear choice for scratch and wear resistance. |
| Corrosion Resistance | Moderate (dependent on porosity and thickness). | Excellent (dense, non-porous structure). | PVD offers a more reliable barrier against environmental degradation. |
| Thickness Range | Wide (typically 1 µm to 50 µm+). | Thin (typically 0.5 µm to 5 µm). | EP allows for thicker, more sacrificial coatings if needed. |
| Environmental Impact | High (involves hazardous chemicals, wastewater treatment). | Low (uses solid targets, minimal waste, no liquid effluent). | PVD aligns better with modern eco-friendly trends in KL manufacturing. |
| CAPEX | Low to Moderate (tanks, rectifiers, ventilation). | High (expensive vacuum chambers, pumping systems). | EP is easier to set up; PVD requires significant initial investment. |
| OPEX | High (chemical replenishment, waste disposal, labour). | Moderate (power consumption, target material replacement). | PVD is often cheaper to run per batch in the long term. |
Environmental and Safety Considerations
In Malaysia, the Department of Environment (DOE) imposes stringent regulations on industrial effluent discharge, particularly concerning heavy metals and cyanide compounds common in certain electroplating baths. This necessitates significant investment in sophisticated wastewater treatment plants and regular compliance audits.
What is the primary environmental advantage of PVD over traditional electroplating? The primary environmental advantage of PVD is that it is a dry process that uses solid source materials, eliminating the need for the large volumes of hazardous chemicals and the subsequent complex wastewater treatment required by electroplating. This significantly reduces the environmental footprint and simplifies compliance with local regulations, making PVD a more sustainable choice for forward-thinking manufacturers.
Quality Assurance and Process Validation
Regardless of the technique chosen, the final product must pass rigorous quality checks. As a technician, I rely on non-destructive testing methods. For both EP and PVD, we use X-ray Fluorescence (XRF) spectrometry to measure coating thickness and composition accurately. Adhesion testing, often via a cross-hatch test or thermal shock, is mandatory. For high-durability items, we subject samples to salt spray testing to validate corrosion resistance. The protocols for these checks are often derived from a universal set of standards, which is why a robust Quality Control checklist is indispensable in any finishing operation.
For PVD, the challenge is often consistency across large batches, requiring precise control of the gas mixture and temperature uniformity within the vacuum chamber. For EP, the challenge is maintaining the chemical balance of the bath and ensuring uniform current distribution across all parts in the jig.
Strategic Application in Corporate Gifting
The choice between EP and PVD ultimately boils down to the end-use and the client's budget.
- High-End, Luxury Items (Watches, Premium Pens, Custom Jewellery): PVD is the preferred choice. The superior hardness and scratch resistance of coatings like black chrome or rose gold TiN ensure the gift maintains its premium look for years. The thin, dense coating preserves the fine details of the underlying substrate, which is critical for intricate laser-engraved logos.
- Mid-Range, High-Volume Items (Keychains, Badges, USB Drives): Electroplating often provides the best cost-performance ratio. A bright nickel or chrome flash over a copper strike offers good aesthetics and adequate durability at a lower unit cost, allowing the client to meet tighter budget constraints for larger campaigns.
- Items Requiring Specific Functional Properties (Conductivity, Solderability): Electroplating, particularly with gold, silver, or tin, remains essential. PVD coatings, while hard, are often non-conductive or semi-conductive, making them unsuitable for electrical contacts or components that require subsequent soldering.
While electroplating is the workhorse of the finishing industry—versatile, cost-effective for volume, and necessary for certain functional requirements—Physical Vapour Deposition represents the future of high-performance, environmentally conscious surface engineering. My role is to evaluate the client's needs against the technical capabilities and limitations of each process, ensuring that the final corporate gift not only looks exceptional but is engineered to last. The capital investment for PVD is substantial, but the long-term benefits in terms of product quality, reduced environmental liability, and superior performance metrics often justify the transition, especially as Malaysian manufacturing continues to move up the value chain.
Planning a Custom Notebook Project?
Check our detailed supplier capabilities guide to see what's feasible for your budget and timeline.